How To Test Garden Soil For Calcium
Fertilizing plants without knowing the soil pH and fertility is like planning a trip without knowing the starting point. You have to know where you are to know what steps to take to get to your destination. You must know the existing pH and fertility to decide how much (if any) lime and fertilizer to apply for optimum plant growth.
Why Test the Soil?
Skip to Why Test the Soil?
Soil testing is a quick and accurate method to determine the relative acidity of the soil (pH) and the level of several essential nutrients (phosphorus, potassium, calcium, magnesium, sodium, sulfur, manganese, copper, and zinc) needed. The test results will aid you in plant selection, soil preparation, and fertilization. They will help you avoid overfertilization, which can stimulate excessive plant growth and increase the likelihood of some diseases. It can also help reduce pollution of our water supplies. Excess nutrients applied, but not used by plants, may run off into surface waters during storms or leach into groundwater. By applying the correct grade and amount of fertilizer, you will avoid unnecessary pruning of excessive new growth and have healthier, more productive plants.
A soil test is the only reliable method to determine soil pH. Most soils in North Carolina are acidic, and some are as acidic as vinegar. Soil pH is a measure of the hydrogen (acid-forming) ion activity of the soil solution. The pH scale of measuring acidity or alkalinity contains 14 divisions known as pH units. It is centered at pH 7, which is "neutral." The lower the number, the more acidic the soil. The higher the number, the more alkaline. The pH scale is not a linear scale but a logarithmic scale. A soil with a pH of 4.0 is 10 times more acidic than soil with a pH of 5.0 and is 100 times more acidic than soil with a pH of 6.0.
Soil pH is influenced by parent material (rock that soil is formed from), precipitation, native vegetation, crops grown, soil depth, and the type and amount of fertilizer used. As organic matter decomposes, acids are produced that leave the soil more acidic. Also, as water from rainfall or irrigation passes through the soil, acids displace basic cations (positively charged ions) such as calcium (Ca) and magnesium (Mg), which are then leached from the soil. Acidity generally increases (pH decreases) with soil depth, so soils that are eroded are acidic unless properly limed. Heavy use of some nitrogen fertilizers also can increase soil acidity.
Soil pH affects the availability of nutrients in the soil as well as those applied as fertilizer (Figure 1). Low pH can cause some elements to become chemically bound to soil particles, which makes them unavailable to plants. Microorganisms responsible for the decay of organic matter may be limited or inactive in highly acidic soil. The ability of legumes to fix nitrogen is also reduced. But when the pH rises above 6.5, trace elements such as iron, manganese, copper, and zinc become less available. The availability of most nutrients is greatest at pH 6.5.
Plants require different pH levels for optimum growth and productivity. A slightly acidic soil (pH 6.0 to 6.5) generally is considered ideal for most plants in North Carolina. However, blueberries, rhododendrons, mountain laurel, and centipedegrass grow best in soils with a pH between 5.0 and 5.5. If the soil pH is above the preferred range for a plant, growth will be slowed or the plant may develop stress problems, such as diseases, insects, nutrient deficiency symptoms, and dieback.
Factors Affecting Soil pH
Soil pH is influenced by parent material (rock that soil is formed from), precipitation, native vegetation, crops grown, soil depth, and the type and amount of fertilizer used. As organic matter decomposes, acids are produced that leave the soil more acidic. Also, as water from rainfall or irrigation passes through the soil, acids displace basic cations (positively charged ions) such as calcium (Ca) and magnesium (Mg), which are then leached from the soil. Acidity generally increases (pH decreases) with soil depth, so soils that are eroded are acidic unless properly limed. Heavy use of some nitrogen fertilizers also can increase soil acidity.
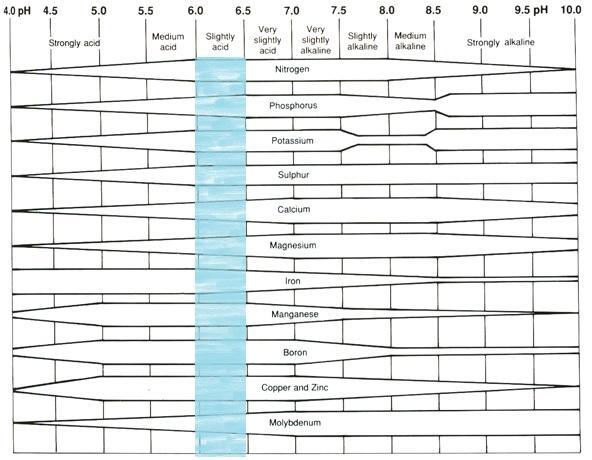
Figure 1. Nutrient availability as affected by soil pH. The wider areas represent greater availability.
How To Test Soil
Skip to How To Test Soil
Most inexpensive, commercially available soil test kits are not reliable. Even if they accurately measure pH, they do not indicate the amount of lime needed. Soil texture, organic matter content, crop to be grown, target pH, soil acidity level, cation exchange capacity (CEC), type and amount of clay, and current pH are factors to consider in determining the amount of lime needed to raise the soil pH.
Consistently reliable results can only be obtained by submitting samples to a soil-testing laboratory. The North Carolina Department of Agriculture and Consumer Services Agronomic Division will analyze your soil samples free of charge or for a small fee. Forms and boxes for samples are available from your local county Cooperative Extension center.
When and how often should soil be tested?
Soils samples may be taken any time of the year. The pH and phosphorus level are relatively constant throughout the year unless lime, fertilizer, or organic matter has been applied recently. It's best not to sample immediately after applying lime, fertilizer, compost, or manure.
The soil-test report will make recommendations for the next growing season, so test soil several months before planting or fertilizing. For a cool season lawn, submit samples the previous summer; for a warm-season lawn, submit samples in the fall or winter. For a spring vegetable garden or flower bed, submit a sample in the fall or winter.
If the soil-test report indicates the pH and nutrient levels are in the range needed for plants to be grown, you may not need to sample every year. If the levels are excessively high or low, you should submit a sample every year to determine how much improvement has been achieved and what additional amendments should be made. As a general rule, test sandy soils every two to three years and clayey soils every three to four years.
How to take a soil sample
The accuracy of the soil-test report depends on the quality of the soil sample. It is best to collect soil samples with stainless steel or chrome-plated tools. The best tool to use is a soil coring tool. It takes an equal amount of soil from the surface through the sampling depth and an equal amount from each site. A shovel or trowel can be used if a coring tool is not available. Avoid using brass, bronze, or galvanized tools, which may contaminate the sample with copper and/or zinc. Put the sample in a clean, plastic bucket; even small amounts of residual lime or fertilizer will affect test results (Figure 2).
Scrape leaves, mulch, and other debris from the soil surface. When using a trowel or shovel, dig a hole to the appropriate depth for each type of plant to be grown, then scrape soil from the side of the hole—one stroke, bottom to top. For gardens, new lawns, and other cultivated areas, sample to the depth the soil has been or will be tilled. For established lawns, collect samples 4 inches deep. For vegetable gardens and flower beds, take samples 6 to 8 inches deep, and for trees and shrubs, sample to a depth of 6 to 10 inches.
Repeat this procedure in six to eight areas (subsamples) and combine to obtain a more representative sample for testing.
Avoid areas that are obviously different—wet spots, the compost pile, animal urine spots, brush piles, under eaves, and sites where trash has been burned. Mix the subsamples together to obtain one composite sample. Remove large pieces of organic material such as roots, stalks, leaves, rocks, and other debris. Fill the soil sample box to the fill line. Submitting less than the suggested amount of soil could lead to a sample that does not adequately represent the area you are testing or may not produce enough soil to conduct all of the necessary tests .
Even if the soil looks the same, take separate samples for each general type of plant to be grown-flower beds, vegetable gardens, fruit orchards, shrub borders, and lawn areas. It's not necessary to list each plant to be grown. But if only one type of plant is to be grown, be specific; for example, list fescue for lawns and apples instead of fruit trees. You will receive a report for each area. Areas with different soil types should be sampled separately. Soils vary by location, slope, the amount of fertilizer applied in recent years, and in their physical, chemical, and biological properties. Soil variations can also result on a new home site when soil is moved around or brought in to fill low areas.
Soil moisture will not affect soil-test results directly. However, it's best if the soil is slightly damp to dry-not wet when samples are collected. If the soil is too wet to till, it's too wet to sample. Wet soil is more difficult to mix and could damage the soil sample box during shipment.
Problem Area Samples
If you have plants in one area that are not doing well, submit a problem area soil sample. Take one soil sample from the problem area and another from a good area for comparison. Fill out the Diagnostic Soil Sample Information Sheet (Form AD2) instead of the regular soil sample sheet.
Completing the soil report form
Enter your name, address, and a five-digit code that you make up on the side of the box using a ballpoint pen, permanent marker, or a No. 2 pencil. Felt tip pens or hard pencils may be difficult to read at the soil-testing lab-especially if the soil box gets wet. Make up a code that will be easy to remember—"lawns" for lawn, "veggie" for vegetable garden. Any combination of letters and numbers may be used. Fill out the soil-test report sheet, giving as much information as possible. The required items are name, address, county, crop code (found on the back of the sheet), and the crop (plants) to be grown. The form is also used by farmers, so some of the information requested, such as pounds of lime per acre, may not seem to apply to gardeners.
Fold the report form so it can be inserted between the flaps in the top of the sample box or tape it to the sample box. Do not put the information sheet inside the sample box.
There is no need to tape the bottom of the box to prevent soil from sifting out. If more than one sample is to be sent, place all samples in a paper bag or cardboard box. Placing soil or boxes in a plastic bag will prevent the soil from air-drying and will take longer to analyze. M ail your sample to the NCDA&CS lab at 5300 Reedy Creek Road in Raleigh.

Figure 2. Use a coring tool or trowel to collect soil samples and place them into a clean bucket before transferring them to a soil sample box.
Karen Neill
Soil Test Results
Skip to Soil Test Results
After the soil-testing lab receives your sample, it dries the soil and conducts tests to determine the soil pH, humic matter content (the chemically active portion of organic matter), nutrient content, and exchange capacity (ability to hold nutrients). The lab chemically removes elements from the soil and measures them for their plant availability. The quantity of available nutrients in the sample, except for nitrogen, is used to determine the amount of fertilizer that will be recommended.
Test results and suggested lime and fertilizer application rates will be posted on the Agronomic Services Division's Public Access Laboratory-information-management System (PALS). The turnaround time is about two weeks during the summer and several months in late fall or early winter.
The report has two sections-test results and lime and fertilizer recommendations. The test results section includes measurements of pH, phosphorous (P), and potassium (K). The Recommendations sections will provide guidance for applications of lime as well as N-P-K fertilizer.
Understanding soil-test report terms
Soil class : Each soil sample is classified according to humic matter content. The classes are:
MIN: Mineral soil. Low percentage of humic matter. Target pH 6.0.
M-O: Mineral-organic soil. Medium percentage of humic matter. Target pH 5.5.
ORG: Organic soil. High humic matter content. Target pH 5.0.
Target pH is the soil pH that is best for most plants. Mineral soils need to be limed to a higher pH than the two other types of soil to reduce aluminum (Al) to levels that will allow optimum growth. Mineral-organic and organic soils are higher in organic matter and lower in aluminum.
HM percent : Humic matter percent is a measure of the chemically active fraction of organic matter. The humic matter values are usually much lower than the actual organic matter content.
W/V : The soil weight/volume is shown in grams/cubic centimeter and is used to determine the soil class. Soils high in sand have high W/V, while soils high in organic matter have low W/V. Loamy and clayey soils are intermediate.
CEC : Cation exchange capacity is a measure of the soil's capacity to hold basic cations such as potassium, calcium, and magnesium, plus the acidic cations hydrogen and aluminum. CEC increases as soil organic matter, pH, and clay content increase. This calculation is given in milliequivalents per 100 grams of soil. Cations are positively charged ions such as calcium (Ca++), magnesium (Mg++), and potassium (K+). The larger the CEC value, the more cations the soil is able to hold against leaching.
BS% : Base saturation percent is the percent of the CEC that is occupied by the basic cations [potassium (K), calcium (Ca), and magnesium (Mg)]. BS% indicates the pH and lime status of the soil. As pH increases, BS% also increases. On soils that are properly limed, BS% should range from 70 to 90. On acidic soils, BS% ranges from 50 to 60.
Ac : Exchangeable acidity is the portion of the CEC that is occupied by acidic cations [Aluminum (Al), hydrogen (H)]. The amount of acidity decreases as soil pH increases.
pH : Soil pH is a measure of the active acidity [hydrogen (H)] in the soil solution.
P-1 and K-1 : Phosphorus (P) and potassium (K) are shown as indexes used to evaluate nutrient availability to plants. Fertilizer recommendations for P and K decrease as the index increases. An index of 25 or lower is considered too low for optimum plant growth. A range of 26 to 50 is medium, and an index of greater than 50 is high. Adding more phosphorus when the index is greater than 50 should not generate a response. Fertilizer rates are given as pounds of P2O5 and K20 per acre or per 1,000 square feet.
Ca and Mg% : Both calcium (Ca) and magnesium (Mg) are shown as percentages of CEC. Soil calcium is seldom low enough to limit plant growth. In general, calcium is the most common cation in the soil. Calcium percentage is essential for calculating CEC and to evaluate the relationship between calcium, magnesium, and potash (K). If the magnesium percent is low, magnesium will be recommended in the form of dolomitic lime or of a fertilizer containing magnesium.
S (sulfur), Mn (manganese), Zn (zinc), Cu (copper) : An index is determined for each of these nutrients. An index of 25 or lower is considered too low for optimum plant growth. A range of 26 to 50 is medium, and a range of greater than 50 is high. Adding more nutrients should not generate a response when the index is greater than 50. Sulfur is difficult to interpret since, like nitrogen, it leaches readily from sandy soils.
SS-1 : The soluble salt index is a measure of the amount of fertilizer elements and sodium that are soluble in the soil. This test is normally done for greenhouse production and problem area soil samples. A moderate level of soluble salts is desirable, but an excessive amount can injure plants. The degree of injury from soluble salts depends on the soil type, soil moisture, and crop sensitivity.
Na : Sodium is reported as meq/ dm3. Sodium can harm plant growth when it exceeds 15 percent of the CEC. You can leach excessive sodium from the soil by applying gypsum (land plaster).
N (nitrogen) is not routinely a part of the soil-test regimen because the test has limited predictive value. Nitrogen is quite mobile in the soil and may be leached out before planting.
Recommendations for its use are based on the amount of nitrogen normally needed for plant growth in a year.
Lime and fertilizer recommendations
When the soil pH is in the ideal range for optimum plant growth, no lime recommendation is given. If the pH was determined to be too low, a recommendation is made to apply lime at a given rate per M. The M stands for 1,000 square feet. Occasionally, the recommendation is given in tons per acre. An acre is 43,560 square feet, and a ton of lime weighs 2,000 pounds. One ton per acre equals 46 pounds per 1,000 square feet.
Sometimes soils with an identical pH will have different lime recommendations. Soils low in organic matter or high in sand require less lime to change the pH than clayey soils or those with high organic matter. Clayey soils contain more potential acidity than sandy soils. As the pH falls below 5.5, aluminum becomes soluble at levels toxic to plants. In addition, soluble aluminum reacts with water to produce hydrogen ions, further reducing soil pH. The purpose of liming is to reduce exchangeable aluminum to levels that are not toxic to plants.
Calculating the Amount of Lime and Fertilizer to Apply
A 1,000-square-feet area is an area 50 feet by 20 feet. Multiply the length of the area by the width of the area to determine the number of square feet. Divide by 1,000 to obtain the number of units to be treated. Multiplying the number of units by the pounds of material to treat 1,000 square feet will give you the amount of fertilizer and lime needed.
Example:
If the area is 500 feet by 20 feet, and the suggested lime or fertilizer treatment is 30M (pounds per 1,000 square feet):
500 feet × 20 feet = 10,000 square feet
Divide 10,000 square feet by 1,000 = 10 units
Multiply 30 pounds by 10 units = 300 pounds of material (fertilizer or lime)
Liming to raise soil pH
Two general classes of liming material may be used to raise the soil pH. Calcitic lime is composed of calcium carbonate and can be used on soils high in magnesium. Dolomitic lime is a mixture of calcium and magnesium carbonates; it should be used on soils low in magnesium. Many organic soils and some piedmont soils are naturally high in magnesium, while most sandy soils in the coastal plain are low in magnesium. Dolomitic lime provides the major portion of calcium and magnesium required for plant growth. Gypsum, also called land plaster, is calcium sulfate. It is an economical source of calcium and sulfur, but it does not affect soil pH.
All limestone sold in North Carolina must have a label showing the guaranteed percentage of calcium, magnesium, and calcium carbonate equivalent, as well as the pounds of material that equal 1 ton of standard lime.
Lime can be purchased in powder or pellet form. The finer the powder, the more rapidly it becomes effective. Pelletized lime contains finely ground dolomitic lime bound into pellets. The pellets disintegrate and release the lime when they contact water. It is usually more expensive, but easier and less messy, to apply pelletized lime than powdered lime. The lime will act more quickly if the soil is retilled several days after the pellets have been mixed into the soil and have had time to soften.
Changing the soil pH
If the soil pH is too acidic, lime can be used to raise the pH. It can be applied any time of the year. Lime raises the pH, providing a more favorable environment for soil microorganisms. Also, plants utilize fertilizers more effectively at the proper pH. Ideally, lime should be applied and incorporated into the soil before planting.
If the soil pH is too alkaline for the plant to be grown, lower the soil pH by incorporating an acidic soil amendment such as pine bark or peat moss or by applying elemental sulfur. Apply sulfur with caution since applying too much can harm plants.
Lime must be mixed with acidic soil and have adequate water to react with the soil. To be effective, lime should be spread and thoroughly incorporated. Lime is only slightly soluble in water and does not move into soil as effectively as soluble fertilizers. With adequate moisture, lime begins to react immediately; however, it can take 6 to 12 months to realize the total benefit from lime.
Surface-applied lime reacts more slowly than lime incorporated into the soil. However, a surface application is better than no application. Most of the surface-applied lime stays in the top 1 to 2 inches of soil. For established lawns, gardens, and ornamentals, up to 50 pounds of lime per 1,000 square feet can be surface applied in one application. For rates over 50 pounds, wait several months to make a repeat application. In lawns, it's best to aerate the soil before applying lime.
Substituting different grades of fertilizer
The soil-test report gives recommendations for a rate and grade of fertilizer to apply per 1,000 square feet. One grade of fertilizer can be substituted for another, but you will need to make a few calculations. For example, when the report recommends 10 pounds of 10-10-10 to apply 1 pound of nitrogen per 1,000 square feet but you want to use a 15-15-15 fertilizer, use the following formula:
Pounds of nitrogen desired per 1,000 square feet ÷ Percentage of nitrogen in fertilizer you plan to use divided by 100 = 1 ÷ (15 ÷ 100) = 1 ÷ .15 = 6
When the soil has a high phosphorus index (P-I), the report may recommend an unusual fertilizer grade such as 15-0-14 or 8-0-24. A fertilizer that contains a small amount of phosphorus (the middle number in the fertilizer analysis) can be substituted for a fertilizer grade that may be next to impossible to find. When the phosphorus index is below 25, a fertilizer with a high phosphorus content is recommended. An alternative method to apply adequate phosphorus is to use a high phosphorus fertilizer, such as 0-46-0, and a conventional fertilizer, such as 10-10-10.
Some fertilizer recommendations pertain to nitrogen only, such as 1 pound of actual nitrogen per 1,000 square feet instead of pounds of a complete fertilizer. This type of recommendation usually is given when the P and K indexes are over 50. To determine the amount of fertilizer to use when only nitrogen is recommended, divide 100 by the first number in a fertilizer analysis (percent nitrogen). For example, if you are using 33-0-0 fertilizer and want to apply 1 pound of actual nitrogen per 1,000 square feet, divide 100 by 33 = 3.3 pounds of actual fertilizer to apply. Table 1 gives the amount of several materials to use if only nitrogen is needed. Unless the soil is deficient in other nutrients, a fertilizer high in nitrogen or containing only nitrogen is often the best buy.
| Application rates per: | |||||
| 1,000 Square Feet | 100 Square Feet | 10 Square Feet | |||
| Source | Pounds | Cups | Pounds | Cups | Tablespoons |
| 10-10-10 | 10 | 20 | 1 | 2 | 4 |
| 8-8-8 | 12.5 | 25 | 1.2 | 2.5 | 5 |
| 12-4-8 | 8 | 16 | .75 | 1.5 | 3 |
| 16-4-8 | 6 | 12 | .5 | 1 | 2 |
| 5-10-10 | 20 | 40 | 2 | 4 | 8 |
| 12-6-6 | 8 | 16 | .75 | 1.5 | 3 |
Acknowlegment
Skip to Acknowlegment
This publication is a revision of an earlier version. The authors would like to thank Erv Evans for his earlier contributions.
Contact Luke Gatiboni for additional information.
Authors
- Lucy Bradley
- Extension Urban Horticulture Specialist
Horticultural Science
- Deanna Osmond
- Department Extension Leader (Nutrient Mgt and Water Quality)
Crop & Soil Sciences
Publication date: Aug. 20, 2019
AG-614
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.
How To Test Garden Soil For Calcium
Source: https://content.ces.ncsu.edu/a-gardeners-guide-to-soil-testing
Posted by: garciajusture70.blogspot.com

0 Response to "How To Test Garden Soil For Calcium"
Post a Comment